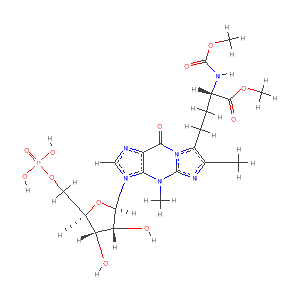
PDB Chemical Component YYG
 |
|
Chemical Description
| Name | 4- |
| Synonyms | MODIFIED GUANOSINE- |
| Formula | C21 H29 N6 O12 P |
| Formal charge | 0 |
| Molecular weight | 588.462 g/mol |
| Component type | RNA LINKING |
| Nonstandard Parent Id | G |
Chemical features
| Atom count | 69 |
| Chiral atom count | 5 |
| Chiral atoms | C4' C3' C2' C1' C15 |
| Observed leaving atoms | OP3 HO3' |
| Bond count | 72 |
| Aromatic bond count | 10 |
Chemical Identifiers
| Systematic name (ACDLabs) | 7- |
| Systematic name (OpenEye OEToolkits) | methyl (2S) |
Chemical Descriptors
| Stereo SMILES (CACTVS) | COC(=O) |
| SMILES (CACTVS) | COC(=O) |
| Stereo SMILES (OpenEye) | Cc1c(n2c(n1) |
| InChI descriptor | InChI=1S/C21H29N6O12P/c1- |
| InChIKey descriptor | WOMRPCAFQVCGCI- |
Status Information
| Last modified | 2020-06-17 |
| Created | 1999-10-04 |
| Release status | REL |
| Model PDB code | 486D |
| Processing site | EBI |